Test to Distinguish Between Aldehydes and Ketones
Test to Distinguish Between Aldehydes and Ketones: Overview
This topic covers concepts, such as, Identification of Aldehydes and Ketones by NaHSO3, Identification of Aldehydes and Ketones by Fehling's Solution & Identification of Aldehydes and Ketones by Benedict's Solution etc.
Important Questions on Test to Distinguish Between Aldehydes and Ketones
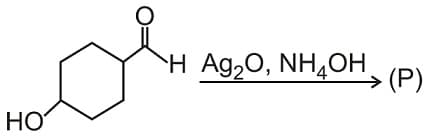
Product (P) is
The Tollen’s regent is:
The chemical tests to distinguish between the following pairs of compounds are:
() Propanal and propanone
() Acetophenone and benzophenone
The following pairs of compounds can be distinguished by which of the following test:
(i) Acetophenone and Benzophenone
(ii) Ethanal and Propanal
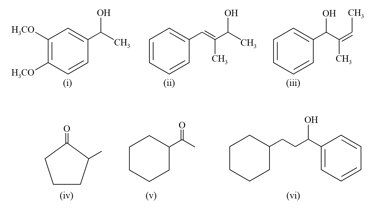
Which compounds among the following give positive iodoform test?
Oxidation of butan-2-one to propionic acid can be achieved by
Which is the major product formed when acetone is heated with iodine and potassium hydroxide?
Glucose on reaction with Fehling solution gives
An organic compound with molecular formula gives an optically active compound on hydrogenation. Upon ozonolysis, produces a mixture of compounds and Compound gives a yellow precipitate when treated with and but does not reduce Tollen's reagent. Compound does not give any yellow precipitate with and but gives Fehling's test. The compound is:
The major products of the following reaction.
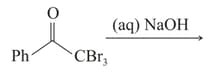 are
are
A mixture of acetaldehyde and acetophenone can be separated by using___________
Which of the following react with and form precipitate.
For next two question please follow the same
Five isomeric p-substituted aromatic compound (A) to (E) with molecular formula C8H8O2 are given for identification. Based on the following observation, give the structures of the compounds.
i. Both (A) and (B) form a silver mirror with Tollen's reagent. (B) also gives a positive test with neutral FeCl3 solution.
ii. (C) gives positive iodoform test.
iii. (D) is readily extracted in aqueous NaHCO3 solution.
iv. (E) on acid hydrolysis gives 1, 4-dihydroxybenzene.
Compound (B) is :
When ethanal is heated with Fehling's solution, then the precipitate obtained is of:
The compound is tested by following tests and their result are:
| Test | Inference |
| - test | Coloured precipitate yellow |
| Iodoform test | Yellow precipitate |
| Azo-dye test | No dye formation |
What is the Compound ?
Among the following compounds, which can give black shiny surface and little bit smell of ammonia when treated with silver diammine complex in basic medium?
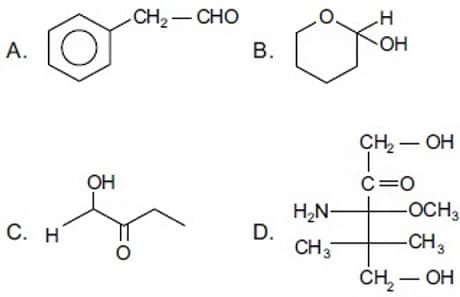
Select the compound which will give a positive iodoform test.
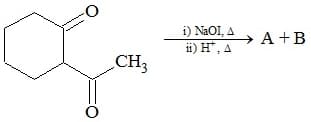
What are the end products A and B of the following sequence of reaction?
On treatment with I2 and NaOH, An aromatic compound give 2 moles of and compound . After the acidification, compound U gives 2 mononitro products on nitration. Compound (T) can also be obtained by the process of ozonolysis of V, in this ozonolysis 1 mole of is obtained along with (T). Which of the following statement is not correct?
Positive iodoform test will not be given by:
